Overseas R & D Centre
Overview
Set up in 2013, Overseas R & D Centre is a continuously growing department of Overseas Health Care Pvt. Ltd. It has its major focus on developing pharmaceutical and nutraceutical products complying to the applicable regulatory guidelines in order to ensure that a quality product is delivered to its consumer.
Well defined set up established in 2013.
Development of Quality based brands complying to regulatory guidelines
We have a technically competent team who has expertise in their respective fields and together we aim to develop concept based brands supported by scientific research and ensure their international quality which can provide our team a competitive edge in the ever growing pharmaceutical and nutraceutical market. Till now we have developed more than 70 products in pharmaceutical and nutraceutical segments, out of which more than 50 products are currently available in domestic market.
Technically competent team
Developed more than 70 products
50 + products in domestic market
As a contribution to the scientific community, our team has published multiple research and review papers in reputed scientific journals. Our team expertise can also be proven from the fact that we contribute as an expert reviewer for multiple leading scientific journals.
Published multiple scientific papers
Serving as an expert reviewer for reputed scientific journals
As a research-led company, we believe in practicing the highest standards of integrity. We bring scientific rigor to all stages of product development from product conception and development to its manufacturing and supporting our marketing team. With complete adherence to quality guidelines, we work collaboratively with academic backbone to undertake fundamental research that helps us address unmet conditions and improve public health.
Research driven, work with high standards of integrity
Actively collaborate with academic backbone for research advancement
Highly motivated to improve public health
Products Facilities Available
We have all the necessary infrastructure required for the development of

Oral Solid Dosage Forms
Tablets
- Fast disintegrating tablet
- Immediate release tablets
- Delayed release tablets
- Sustained release tablets
- Bilayer tablets
Capsules
- Hard gelatin capsule (including tablet in capsule technology)
Oral Liquid dosage forms
- Syrups
- Suspensions
Quality Compliance
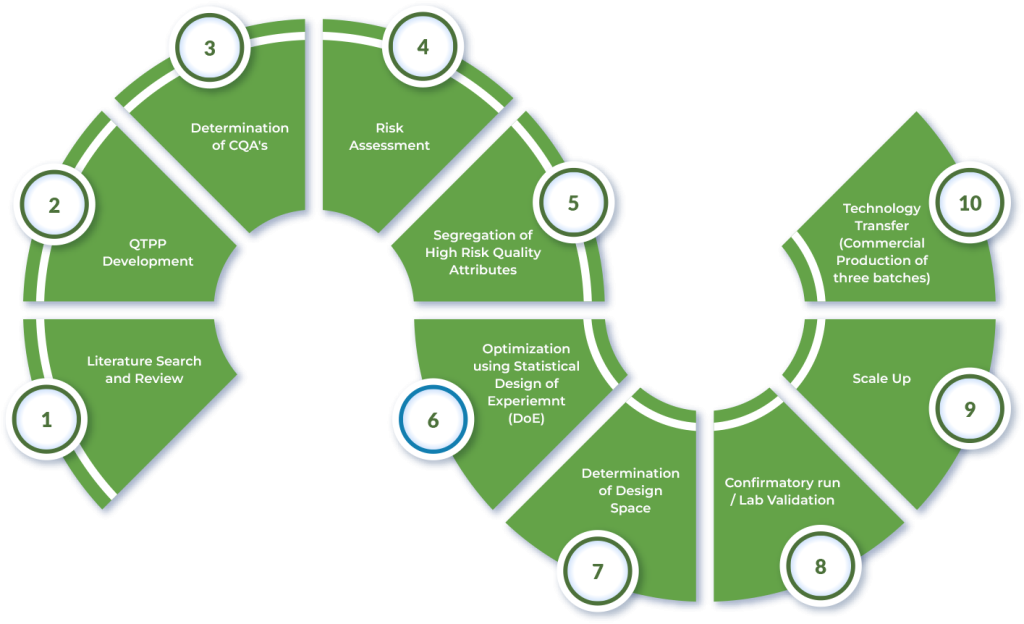
Work in Overseas R & D Center is being done as per standard quality product development guidelines (including ICHQ8). A brief layout of our work methodology is as follow:
New Product Development in Overseas R & D Center
Achievements
Set up in 2013, Overseas R & D Centre is a continuously growing department of Overseas Health Care Pvt. Ltd. It has its major focus on developing pharmaceutical and nutraceutical products complying to the applicable regulatory guidelines in order to ensure that a quality product is delivered to its consumer.
- Have developed more than 70 products till now, out of which more than 50 products are currently available in Indian market
- Published multiple original, reviews, commentary and meta-analysis research in various reputed scientific journals. List of Overseas R & D Centre’s publications is mentioned below:
Verma H, Garg R. 2020. Evaluation of synergistic combination comprising magnesium orotate, menaquinone-7, and cholecalciferol for management of type 2 diabetes and dyslipidemia, Magnesium Research, 33: 88 – 105.
Verma H, Garg R. 2020. Study of Degradation Kinetics of Magnesium Orotate Dihydrate by Spectroscopic Method, Journal of Applied Spectroscopy, 87: 505 – 514.
Garg R, Kaur S, Ritika, Khatoon S, Naina, Verma H. 2020. A complete and updated review on various types of drug delivery systems. International Journal of Applied Pharmaceutics, 12: 1 – 16.
Verma H, Garg R. 2016. Comment on “Bone regulates glucose metabolism as an endocrine organ through osteocalcin”, International Journal of Endocrinology, Volume 2016, Article ID 9724929
Verma H, Garg R. 2019. Simultaneous estimation of menaquinone-7 and cholecalciferol in combined pharmaceutical dosage forms by ultraviolet spectrophotometry, Asian Journal of Pharmaceutics, 13: 305 – 312
Verma H, Garg R. 2019. Effect of Vitamin K Supplementation on Cardiometabolic Risk Factors: A Systematic Review and Meta-Analysis, Endocrine, metabolic & immune disorders drug targets, 19: 13 – 25.
Verma H, Garg R. 2017. Effect of magnesium supplementation on type 2 diabetes associated cardiovascular risk factors: A systematic review and meta-analysis, Journal of Human Nutrition and Dietetics, 30: 621 – 633
Verma H, Garg R. 2019. Biopharmaceutics classification and pharmacokinetics study of magnesium orotate, Magnesium Research, 32: 132 – 142.
Verma H, Garg R. 2015. Comment on “The effect of chromium picolinate supplementation on the pancreas and macroangiopathy in type II diabetes mellitus rats”, Journal of Diabetes Research, Volume 2015, Article ID 901895.
Verma H, Garg R. 2015. Comment on “Alpha-Lipoic Acid and Antioxidant Diet Help to Improve Endothelial Dysfunction in Adolescents with Type 1 Diabetes: A Pilot Trial”, Journal of Diabetes Research, Volume 2015, Article ID 646095.
Verma H. 2015. Comment on “Effect of pumpkin seed oil on hair growth in men with androgenetic alopecia: a randomized, double-blind, placebo-controlled trial”, Evidence-Based Complementary and Alternative Medicine, Volume 2015, Article ID 271474.
Verma H, Gupta B. 2013. Bioequivalence study of isoxsuprine HCl capsules in healthy Indian volunteers, International Journal of Pharmacology & Toxicology Science, 3: 17-23
Verma H, Gupta B. 2013. Bioequivalence study of fixed dose combination of rabeprazole (EC) and domperidone ((SR) in healthy Indian









Serve as expert reviewer for following journals:
Biological Trace Element Research
Diabetes, Obesity and Metabolism
The Journal of Nutrition
Journal of Diabetes and Metabolic Disorders
Evidence Based Complementary and Alternative Medicine
Phytotherapy Research





Patent

Title of Patent:- “Vitamin and Mineral Composition for the Treatment of Type 2 Diabetes and Method Thereof.”
Current Status:- Published, under amended examination
